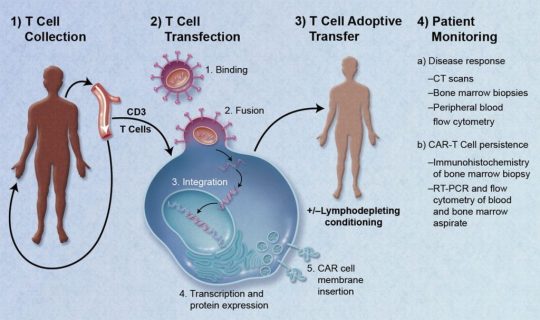
#car t-cell therapy
Explore tagged Tumblr posts
Text
Pediatric Hematology-Oncology: Advances in Childhood Cancer Treatment
With time, many recent advances and developments have been made in Pediatric Hematology and oncology. Students often are short of time to track such recent findings, which can prevent them from becoming the best medical professionals.
If we look at this branch of MD, we can see that many new ways of treating cancer have entered the field. All of these help children battle cancer easily. To make you aware of such recent developments, we are writing this blog post.
Therefore, as a postgraduate, you must be aware of what is currently happening in the field of Pediatric Oncology.
Latest advances in treating childhood cancer treatment
Over the last few decades, there have been many advances made in the field of Pediatrics and Hematology, which are discussed below:
CAR T Cell Therapy:
If you want to know about how to prevent childhood cancer with the newest and most personalized treatment, then CAR T Cell Therapy has all your answers. This Chimeric Antigen Receptor (CAR) T Cell Therapy is for kids who get leukemia back even after treatment.
Radiation therapy:
Although Radiation therapy is another childhood cancer treatment, yet again, it is the talk of the town because newer and more precise ways of delivering radiation are being developed. In this therapy, cancer cells are destroyed with the help of high-energy X-rays, protons, and photons. It includes both external and internal radiation therapy.
Precision medicine:
The scope of treating childhood cancer goes beyond the universally applicable treatments mentioned above. In this approach, genetic analysis can find out the mutations causing a child’s cancer, which allows doctors to customize their treatment, which gives fewer side effects than chemotherapy.
Liquid biopsy:
Another type of development made in childhood cancer treatment is Liquid biopsy. This minimally invasive technique allows doctors to quickly identify a kid's cancer cells. They do this by analyzing tumor DNA found in their blood. This can help them to give better treatment to the children when the cancer relapses again.
By reading these discoveries, we can see that children can overcome cancer and come back to living healthier and happier lives than they used to live earlier.
The following section is designed for postgraduate students who want to learn more about this field.
Pediatrics MD— Course
This Pediatrics MD course at DigiNerve, designed by Dr. Piyush Gupta, helps students get all the latest and resourceful information they can't get elsewhere due to their time shortage.
By enrolling in this MD in Pediatrics, students can get a range of benefits such as:
Online video lectures: The course includes 170+ hours of pediatrics video lectures.
Self-assessment questions: If you want to practice yourself and know where you stand, then you can try 1490+ MCQ questions outlined in the course.
Engagement activities: There are a range of activities, such as chat shows, journal clubs, and recent updates on the field.
AI chatbot: Dr. Wise (AI chatbot) can help you clarify all your theoretical and practical concepts.
So, if you want to stay updated with the latest information and advances in Pediatric Oncology, buy this course now!
Conclusion
Staying informed with the help of resources like this MD course led by qualified professionals like Dr. Piyush Gupta, you can pave the way towards becoming a medical professional and assist in creating a better and healthier future for children.
#Pediatrics MD#MD in Peditrics#Dr. Piyush Gupta#Online Pediatrics course#Pediatrics Video Lecture#CAR T-Cell therapy#how to prevent childhood cancer#childhood cancer#childhood cancer treatment#pediatric oncology
2 notes
·
View notes
Text
Immuneel launches affordable CAR T-cell therapy for non-Hodgkin’s Lymphoma in India

Bengaluru-based cell and gene therapy start-up Immuneel Therapeutics on Monday announced the launch of Qartemi — the country’s first personalised and precision therapy CAR T-cell therapy for treating B-cell Non-Hodgkin Lymphoma (B-NHL) in adults.
Source: bhaskarlive.in
0 notes
Text
A new way to miniaturize cell production for cancer treatment
New Post has been published on https://thedigitalinsider.com/a-new-way-to-miniaturize-cell-production-for-cancer-treatment/
A new way to miniaturize cell production for cancer treatment


Researchers from the Singapore-MIT Alliance for Research and Technology (SMART), MIT’s research enterprise in Singapore, have developed a novel way to produce clinical doses of viable autologous chimeric antigen receptor (CAR) T-cells in a ultra-small automated closed-system microfluidic chip, roughly the size of a pack of cards.
This is the first time that a microbioreactor is used to produce autologous cell therapy products. Specifically, the new method was successfully used to manufacture and expand CAR-T cells that are as effective as cells produced using existing systems in a smaller footprint and less space, and using fewer seeding cell numbers and cell manufacturing reagents. This could lead to more efficient and affordable methods of scaling-out autologous cell therapy manufacturing, and could even potentially enable point-of-care manufacturing of CAR T-cells outside of a laboratory setting — such as in hospitals and wards.
CAR T-cell therapy manufacturing requires the isolation, activation, genetic modification, and expansion of a patient’s own T-cells to kill tumor cells upon reinfusion into the patient. Despite how cell therapies have revolutionized cancer immunotherapy, with some of the first patients who received autologous cell therapies in remission for more than 10 years, the manufacturing process for CAR-T cells has remained inconsistent, costly, and time-consuming. It can be prone to contamination, subject to human error, and requires seeding cell numbers that are impractical for smaller-scale CAR T-cell production. These challenges create bottlenecks that restrict both the availability and affordability of these therapies despite their effectiveness.
In a paper titled “A high-density microbioreactor process designed for automated point-of-care manufacturing of CAR T cells” published in the journal Nature Biomedical Engineering, SMART researchers detailed their breakthrough: Human primary T-cells can be activated, transduced, and expanded to high densities in a 2-mililiter automated closed-system microfluidic chip to produce over 60 million CAR T-cells from donors with lymphoma, and over 200 million CAR T-cells from healthy donors. The CAR T-cells produced using the microbioreactor are as effective as those produced using conventional methods, but in a smaller footprint and less space, and with fewer resources. This translates to lower cost of goods manufactured (COGM), and potentially to lower costs for patients.
The groundbreaking research was led by members of the Critical Analytics for Manufacturing Personalized-Medicine (CAMP) interdisciplinary research group at SMART. Collaborators include researchers from the Duke-NUS Medical School; the Institute of Molecular and Cell Biology at the Agency for Science, Technology and Research; KK Women’s and Children’s Hospital; and Singapore General Hospital.
“This advancement in cell therapy manufacturing could ultimately offer a point-of-care platform that could substantially increase the number of CAR T-cell production slots, reducing the wait times and cost of goods of these living medicines — making cell therapy more accessible to the masses. The use of scaled-down bioreactors could also aid process optimization studies, including for different cell therapy products,” says Michael Birnbaum, co-lead principal investigator at SMART CAMP, associate professor of biological engineering at MIT, and a co-senior author of the paper.
With high T-cell expansion rates, similar total T-cell numbers could be attained with a shorter culture period in the microbioreactor (seven to eight days) compared to gas-permeable culture plates (12 days), potentially shortening production times by 30-40 percent. The CAR T-cells from both the microfluidic bioreactor and gas-permeable culture plates only showed subtle differences in cell quality. The cells were equally functional in killing leukemia cells when tested in mice.
“This new method suggests that a dramatic miniaturization of current-generation autologous cell therapy production is feasible, with the potential of significantly alleviating manufacturing limitations of CAR T-cell therapy. Such a miniaturization would lay the foundation for point-of-care manufacturing of CAR T-cells and decrease the “good manufacturing practice” (GMP) footprint required for producing cell therapies — which is one of the primary drivers of COGM,” says Wei-Xiang Sin, research scientist at SMART CAMP and first author of the paper.
Notably, the microbioreactor used in the research is a perfusion-based, automated, closed system with the smallest footprint per dose, smallest culture volume and seeding cell number, as well as the highest cell density and level of process control attainable. These microbioreactors — previously only used for microbial and mammalian cell cultures — were originally developed at MIT and have been advanced to commercial production by Millipore Sigma.
The small starting cell numbers required, compared to existing larger automated manufacturing platforms, means that smaller amounts of isolation beads, activation reagents, and lentiviral vectors are required per production run. In addition, smaller volumes of medium are required (at least tenfold lower than larger automated culture systems) owing to the extremely small culture volume (2 milliliters; approximately 100-fold lower than larger automated culture systems) — which contributes to significant reductions in reagent cost. This could benefit patients, especially pediatric patients who have low or insufficient T-cell numbers to produce therapeutic doses of CAR T-cells.
Moving forward, SMART CAMP is working on further engineering sampling and/or analytical systems around the microbioreactor so that CAR-T production can be performed with reduced labor and out of a laboratory setting, potentially facilitating the decentralized bedside manufacturing of CAR T-cells. SMART CAMP is also looking to further optimize the process parameters and culture conditions to improve cell yield and quality for future clinical use.
The research was conducted by SMART and supported by the National Research Foundation Singapore under its Campus for Research Excellence and Technological Enterprise (CREATE) program.
#Analytics#antigen#author#Biological engineering#Biology#Biomedical engineering#Cancer#cancer treatment#CAR T-cell therapy#cell#cell biology#cell cultures#cell therapies#cell therapy#Cells#Chemical engineering#Children#contamination#engineering#enterprise#Foundation#Future#gas#genetic#Health sciences and technology#hospitals#how#human#immunology#immunotherapy
0 notes
Text
youtube
#Checkpoint inhibitors#CAR T-cell therapy#Cytokines#Cancer vaccines#Monoclonal antibodies#Oncolytic virus therapy#PD-1 inhibitors#CTLA-4 inhibitors#Immune response#Tumor microenvironment#Autoimmune reactions#T-cells#Interleukins#Youtube
0 notes
Text
The global CAR T-Cell Therapy Market is projected to reach more than USD 22.2 billion by 2032 from USD 2.1 billion in 2023, growing at a CAGR of 30% from 2024-2032.
Key Players in Global CAR T-Cell Therapy Market
Novartis AG
Bluebird Bio, Inc.
Cellectis
Bristol-Myers Squibb
Merck & Co., Inc.
Juno Therapeutics, Inc.
Celyad Oncology
Celgene Corporation
Sorrento Therapeutics, Inc.
Miltenyi Biotech
Intellia Therapeutics
Pfizer, Inc.
Autolus Therapeutics
Gilead Sciences, Inc. (Kite Pharma Inc.)
Cartesian Therapeutics, Inc.
Caribou Biosciences, Inc.
Other Prominent Players
0 notes
Text
Can Immunotherapy Revolutionize Cancer Treatment?
Cancer is a relentless foe, affecting millions of lives worldwide. Conventional cancer treatments like chemotherapy and radiation therapy have been the primary choices for years, but they often come with severe side effects and limitations. Is there a more effective and less invasive way to combat this deadly disease? That's where cancer immunotherapy comes into play. In this comprehensive guide, we will explore the groundbreaking advances in cancer immunotherapy and the challenges it faces, as well as how online resources can enhance the cancer therapy process.
#cancer treatment#immunotherapy#cancer therapy#cancer immunotherapy#CAR T-Cell Therapy#online doctor consultation#online lab test
0 notes
Text
Latest Pharma News: Walgreens, UT Tyler’s Fisch College & CAR T-Cell Therapy
Latest Pharma News: Latest Research Developments in Pharmacy for 2023: The field of Pharmacy is continuously evolving, with groundbreaking research shaping the future of healthcare. This article aims to provide a comprehensive overview of the latest research developments in Pharmacy for the year 2023. Latest Pharma News: Walgreens, UT Tyler’s Fisch College & CAR T-Cell Therapy Walgreens and…

View On WordPress
0 notes
Text
0 notes
Text
They may have found a way to cure autoimmune diseases. It is called CAR-T Cell Therapy.
3 notes
·
View notes
Text
instagram
6 notes
·
View notes
Text
India approved a living drug to treat Blood Cancer
India approved a living drug to treat Blood Cancer @neosciencehub #India #BloodCancer #CDSCO #livingdrug #Qartemi #neosciencehub
For Indian patients with blood cancer who are at an advanced or relapsed stage of the disease, a “living drug” has been licensed. Patients with B-cell Non-Hodgkin Lymphoma (B-NHL) can now receive Qartemi, a CAR-T cell therapy, thanks to Immuneel Therapeutics, a biotech business based in Bengaluru. After the Central Drugs Standard Control Organization (CDSCO) authorized the indigenous NexCAR19,…
#Blood Cancer#CAR-T cell therapy#Central Drugs Standard Control Organization (CDSCO)#featured#India#Qartemi#sciencenews
0 notes
Link
#pioneering marketdigits consulting and advisory private limited#therapy medicinal products#advanced therapy medicinal#cell therapy#car-t therapy
0 notes
Text
CAR T-Cell Therapy Market to Hit $5.9 Billion by 2032
What's Trending in CAR T-Cell Therapy Market?
- Keep Yourself Up-To-Date With The Latest Market Trends.
The global CAR T-Cell Therapy Market was valued at USD 2 Billion in 2024 and it is estimated to garner USD 5.9 Billion by 2032 with a registered CAGR of 14.6% during the forecast period 2024 to 2032.
Firstly, the Market report for CAR T-Cell Therapy Market describes the current state of the companies and recommends where it is likely to go next. The report shows the production, revenue, price, market share, and growth rate of each type, mainly divided into Product Types and Product Applications etc.
Additionally, this market report focuses on offering key business measures such as real market moves, market size, qualities, and freedoms, as well as forecast opportunities. This CAR T-Cell Therapy Market report also offers distinctive insights into wealthy regions such as Europe, North America, the Middle East, Africa, and Latin America.
Get a Sample Copy of the CAR T-Cell Therapy Market Report at: https://www.vantagemarketresearch.com/buy-now/car-tcell-therapy-market-2336/0
Top Competitors:
Celgene Corporation (U.S.), Cartesian Therapeutics Inc. (U.S.), Miltenyi Biotech (Germany), Autolus Therapeutics (UK), Caribou Biosciences Inc. (U.S.), Gilead Sciences Inc. (U.S.), Merck & Co. Inc. (U.S.), Intellia Therapeutics (U.S.), Juno Therapeutics Inc. (U.S.), Bristol-Myers Squibb (U.S.), Novartis AG (Switzerland), Bluebird Bio Inc. (U.S.), Sorrento Therapeutics Inc. (U.S.)
This market report has all the information you need to start or grow your business in the industry. It also includes market drivers, restraints, competitiveness, and geographic estimates, as well as a price and emerging market structure. It is a complete description of a company's business model, benchmarks, consumer preferences, value proposition, and net profit. This comprehensive CAR T-Cell Therapy Market study also sheds light on key techniques that help companies truly assess their customers' buying behavior.
It represents global economic trends between 2024 and 2032. With the help of this market research, top companies can easily make smarter financial decisions. This market analysis is an excellent technique to help companies implement new products. It also includes critical data on major industry topics, including market expansions and evolving market conditions.
This well-researched CAR T-Cell Therapy Market report describes the negative impact COVID-19 is having on various companies and offers companies recommendations on how to recover from the damage suffered by the outbreak as well as the nationwide quarantine. The plan analyzes the company's expectations and priorities, as well as the delivery of all crucial data.
You Can Buy This Report From Here: https://www.vantagemarketresearch.com/buy-now/car-tcell-therapy-market-2336/0
This report analyzes key market segments by type, application, and geography. The geographic analysis section covers key regions such as Europe, North America, the Middle East, Africa, and the Asia-Pacific region.
This CAR T-Cell Therapy Market report not only provides valuable data but outlines key goals, pricing strategies, and approaches to help market participants' recommendations in this report will make accelerating economic growth easy. It offers some specific tips and business-related data to help new competitors in the market grow their businesses and diversify their product lines. Companies in the industry should choose tactics that include new product launches, mergers, and partnerships to survive in the competitive marketplace and strengthen their position.
Regional Analysis
-North America [United States, Canada, Mexico]
-South America [Brazil, Argentina, Columbia, Chile, Peru]
-Europe [Germany, UK, France, Italy, Russia, Spain, Netherlands, Turkey, Switzerland]
-Middle East & Africa [GCC, North Africa, South Africa]
-Asia-Pacific [China, Southeast Asia, India, Japan, Korea, Western Asia]
The quantitative information in this CAR T-Cell Therapy Market analysis helps predict future sales and market penetration. This type of information is based on statistics. The qualitative information provided here will greatly help the key players understand the buyer's opinion of your brand. Improving business goals becomes easy with the information provided in this report.
The industries can draw some conclusions about their original goals. In business. This CAR T-Cell Therapy Market research helps you make assumptions about your competition, customers, and the market in order to make informed business decisions. Additionally, it forecasts the competition in the market for the estimated period of 2024-2032. Effective decision-making in companies leads to business growth and is made possible by this precise market study.
Read Full Research Report with [TOC] @ https://www.vantagemarketresearch.com/industry-report/car-tcell-therapy-market-2336
Some of the Key Questions Answered in this Report:
Which are the five top players of the CAR T-Cell Therapy Market?
How will the CAR T-Cell Therapy Market change in the upcoming years?
Which product and application will take a share of the CAR T-Cell Therapy Market?
What will be the CAGR and size of the CAR T-Cell Therapy Market throughout the forecast period?
What are the drivers and restraints of the CAR T-Cell Therapy Market?
Which regional market will show the highest growth?
What is the current industry size, what will the market size be in 2030 and what will the growth rate be?
Who are the major competitors and what is their strategy?
What are the challenges to grow in the industry?
What are the market opportunities and challenges faced by the key vendors?
What are the barriers to entry for new players in the CAR T-Cell Therapy industry?
Check Out More Reports
Global Smart Thermostats Market: Report Forecast by 2032
Global Dunnage Packaging Market: Report Forecast by 2032
Global Predictive Maintenance Market: Report Forecast by 2032
Global WPA LSG and FT and NMS Market: Report Forecast by 2032
Global Digital Agriculture Market: Report Forecast by 2032
#CAR T-Cell Therapy Market#CAR T-Cell Therapy Market 2024#Global CAR T-Cell Therapy Market#CAR T-Cell Therapy Market outlook#CAR T-Cell Therapy Market Trend#CAR T-Cell Therapy Market Size & Share#CAR T-Cell Therapy Market Forecast#CAR T-Cell Therapy Market Demand#CAR T-Cell Therapy Market sales & price
0 notes
Text
Accelerating CAR T cell therapy: Lipid nanoparticles speed up manufacturing - Technology Org
New Post has been published on https://thedigitalinsider.com/accelerating-car-t-cell-therapy-lipid-nanoparticles-speed-up-manufacturing-technology-org/
Accelerating CAR T cell therapy: Lipid nanoparticles speed up manufacturing - Technology Org
For patients with certain types of cancer, CAR T cell therapy has been nothing short of life-changing. Developed in part by Carl June, Richard W. Vague Professor at Penn Medicine, and approved by the Food and Drug Administration (FDA) in 2017, CAR T cell therapy mobilizes patients’ own immune systems to fight lymphoma and leukemia, among other cancers.
Using activating lipid nanoparticles (aLNPs) to create CAR T cells requires fewer steps and less time.
However, the process for manufacturing CAR T cells itself is time-consuming and costly, requiring multiple steps over days. The state-of-the-art involves extracting patients’ T cells, then activating them with tiny magnetic beads, before giving the T cells genetic instructions to make chimeric antigen receptors (CARs), the specialized receptors that help T cells eliminate cancer cells.
Now, Penn Engineers have developed a novel method for manufacturing CAR T cells, one that takes just 24 hours and requires only one step. This method uses lipid nanoparticles (LNPs), the potent delivery vehicles that played a critical role in the Moderna and Pfizer-BioNTech COVID-19 vaccines.
In a new paper in Advanced Materials, Michael J. Mitchell, Associate Professor in Bioengineering, describes the creation of “activating lipid nanoparticles” (aLNPs), which can activate T cells and deliver the genetic instructions for CARs in a single step, greatly simplifying the CAR T cell manufacturing process. “We wanted to combine these two extremely promising areas of research,” says Ann Metzloff, a doctoral student and NSF Graduate Research Fellow in the Mitchell lab and the paper’s lead author. “How could we apply lipid nanoparticles to CAR T cell therapy?”
In some ways, T cells function like a military reserve unit: in times of health, they remain inactive, but when they detect pathogens, they mobilize, rapidly expanding their numbers before turning to face the threat. Cancer poses a unique challenge to this defense strategy. Since cancer cells are the body’s own, T cells don’t automatically treat cancer as dangerous, hence the need to first “activate” T cells and deliver cancer-detecting CARs in CAR T cell therapy.
Until now, the most efficient means of activating T cells has been to extract them from a patient’s bloodstream and then mix those cells with magnetic beads attached to specific antibodies — molecules that provoke an immune response. “The beads are expensive,” says Metzloff. “They also need to be removed with a magnet before you can clinically administer the T cells. However, in doing so, you actually lose a lot of the T cells, too.”
Made primarily of lipids, the same water-repellent molecules that constitute household cooking fats like butter and olive oil, lipid nanoparticles have proven tremendously effective at delivering delicate molecular payloads. Their capsule-like shape can enclose and protect mRNA, which provides instructions for cells to manufacture proteins. Due to the widespread use of the COVID-19 vaccines, says Metzloff, “The safety and efficacy of lipid nanoparticles has been shown in billions of people around the world.”
To incorporate LNPs into the production of CAR T cells, Metzloff and Mitchell wondered if it might be possible to attach the activating antibodies used on the magnetic beads directly to the surface of the LNPs. Employing LNPs this way, they thought, might make it possible to eliminate the need for activating beads in the production process altogether. “This is novel,” says Metzloff, “because we’re using lipid nanoparticles not just to deliver mRNA encoding CARs, but also to initiate an advantageous activation state.”
Over the course of two years, Metzloff carefully optimized the design of the aLNPs. One of the primary challenges was to find the right ratio of one antibody to another. “There were a lot of choices to make,” Metzloff recalls, “since this hadn’t been done before.”
By attaching the antibodies directly to LNPs, the researchers were able to reduce the number of steps involved in the process of manufacturing CAR T cells from three to one, and to halve the time required, from 48 hours to just 24 hours. “This will hopefully have a transformative effect on the process for manufacturing CAR T cells,” says Mitchell. “It currently takes so much time to make them, and thus they are not accessible to many patients around the world who need them.”
CAR T cells manufactured using aLNPs have yet to be tested in humans, but in mouse models, CAR T cells created using the process described in the paper had a significant effect on leukemia, reducing the size of tumors, thereby demonstrating the feasibility of the technology.
Metzloff also sees additional potential for aLNPs. “I think aLNPs could be explored more broadly as a platform to deliver other cargoes to T cells,” she says. “We demonstrated in this paper one specific clinical application, but lipid nanoparticles can be used to encapsulate lots of different things: proteins, different types of mRNA. The aLNPs have broad potential utility for T cell cancer therapy as a whole, beyond this one mRNA CAR T cell application that we’ve shown here.”
Source: University of Pennsylvania
You can offer your link to a page which is relevant to the topic of this post.
#Administration#advanced materials#antibodies#antigen#Art#bioengineering#Biotechnology news#bloodstream#Cancer#cancer cells#Cancer Therapy#CAR T-cell therapy#Cars#cell#cell therapy#Cells#challenge#Chemistry & materials science news#cooking#course#covid#defense#Design#drug#engineers#FDA#Fight#Food#genetic#Giving
0 notes
Text
👨⚕💉💊🩺 Affordability of CAR-T cell therapy cost in India is generally lower than in many Western countries, primarily due to reduced operational expenses, including labor and infrastructure costs.👨⚕💉💊🩺
#CAR-T Cell Therapy in India#CAR-T Cell Therapy Cost in India#Low Cost CAR-T Cell Therapy in India#Affordable CAR-T Cell Therapy in India#CAR-T Cell for Cancer Treatment in India#CAR-T Cell for Cancer Treatment Cost India#Low Cost CAR-T Cell for Cancer Treatment in India#Affordable CAR-T Cell for Cancer Treatment in India#Best Cancer Hospital for CAR-T Cell in India#Top 10 Cancer Hospital for CAR-T Cell Therapy in India
0 notes
Text
Dr Gaurav Kharya" Advancing Pediatric Cancer Treatment: CAR T Cell Therapy with Dr. Gaurav Kharya"
Dr Gaurav Kharya Best Doctor for Children’s Cancer in Delhi – Redefining Pediatric Oncology with CAR T Cell Therapy
Pediatric cancer treatment has entered a new era, with revolutionary therapies offering more targeted, effective solutions. Among the most promising advancements is CAR T cell therapy, an innovative immunotherapy that is transforming outcomes for children battling cancer. Spearheading this breakthrough in India is Dr. Gaurav Kharya, one of the most trusted and experienced specialists in pediatric oncology, hematology, and immunology. His dedication to providing top-tier care ensures that young patients have access to the latest in cancer treatment.

What Makes CAR T Cell Therapy a Game Changer?
CAR T cell therapy is a form of personalized medicine that reprograms a patient’s T cells to better recognize and eliminate cancer cells. T cells, a type of white blood cell, are extracted from the patient and genetically engineered to express chimeric antigen receptors (CARs). These specialized receptors enable the T cells to locate and attack cancer cells with precision. Once the T cells are reintroduced into the patient’s body, they continuously patrol for cancer cells, even after the primary treatment is complete.
The therapy's ability to target cancer cells while sparing healthy tissues makes it a less invasive option than conventional treatments like chemotherapy and radiation. In many cases, CAR T cell therapy has led to complete remission for patients with certain types of blood cancers, particularly leukemia and lymphoma. This "living drug" approach offers long-lasting protection, as the engineered T cells remain vigilant, significantly reducing the chances of a relapse.
Dr. Gaurav Kharya: A Pioneer in Pediatric CAR T Cell Therapy

As one of the leading doctors for children’s cancer treatment in Delhi, Dr. Gaurav Kharya has made significant contributions to the adoption of CAR T cell therapy in India. With over two decades of experience and more than 1000 successful bone marrow transplants, Dr. Kharya is a renowned expert in pediatric hematology and oncology. His expertise in CAR T cell therapy has allowed many young cancer patients to access this life-saving treatment, providing them with a brighter future.
In his role as the Senior Consultant in Pediatric Hematology, Oncology & Immunology at Apollo Hospitals, Delhi, Dr. Kharya works closely with families to provide tailored, compassionate care. His leadership in immunotherapy and bone marrow transplants ensures that children receive the best possible treatment options, guided by the latest global advancements in cancer care.
The Impact of CAR T Cell Therapy on Pediatric Blood Cancers
CAR T cell therapy has shown remarkable success in treating acute lymphoblastic leukemia (ALL), one of the most common forms of cancer in children, as well as non-Hodgkin lymphoma. These cancers, which often resist traditional treatments, have seen impressive remission rates thanks to CAR T cell therapy. For families facing a pediatric cancer diagnosis, this therapy offers renewed hope, as it targets cancer at its source while minimizing harmful side effects.
Expanding the Reach of CAR T Cell Therapy in India
While CAR T cell therapy is widely recognized and used in the United States and Europe, its availability in India is still growing. However, with experts like Dr. Gaurav Kharya leading the charge, this therapy is becoming increasingly accessible to children across the country. By working with top medical institutions, Dr. Kharya is helping to bring this breakthrough treatment to Indian families, ensuring that they no longer have to travel abroad for advanced cancer care.
Looking to the future, researchers and clinicians like Dr. Kharya are exploring the potential of CAR T cell therapy for treating solid tumors, expanding its use beyond blood cancers.
Why Choose Dr. Gaurav Kharya for Pediatric Cancer Treatment?
When it comes to treating children’s cancers, experience, expertise, and empathy are paramount. Dr. Gaurav Kharya stands as a beacon of hope for families, combining his medical proficiency with a compassionate approach to patient care. His deep involvement in bone marrow transplants, immunotherapy, and CAR T cell therapy positions him as one of the best doctors for children’s cancer in Delhi.
With a strong commitment to staying at the forefront of pediatric oncology, Dr. Kharya’s work continues to change the lives of countless children and their families, offering them the best chance at overcoming cancer.
Conclusion
CAR T cell therapy is reshaping the landscape of pediatric cancer treatment, offering new hope where traditional treatments fall short. Under the expert care of Dr. Gaurav Kharya, children in India now have access to this life-changing therapy. As one of the leading pediatric oncologists in Delhi, Dr. Kharya ensures that each child’s health is prioritized, with personalized, state-of-the-art care that makes a real difference.
#Best Doctor for Children’s Cancer in Delhi#CAR T Cell Therapy for Children#Pediatric Oncology Expert#Bone Marrow Transplant Specialist#Dr Gaurav Kharya#cancertreatment#cancer treatment
0 notes